| Mechanism | Indication | Discovery | Preclinical | Phase 1 | Phase 2 | Phase 3 | Ownership | PIPE-791 |
|---|---|---|---|---|---|---|---|---|
| LPA1R Antagonist | IPF | Wholly-owned | ||||||
| close x | ||||||||
| LPA1R Antagonist | Progressive MS (PrMS) | Wholly-owned | ||||||
| close x | ||||||||
| LPA1R Antagonist | Chronic Pain | Wholly-owned | ||||||
| close x | CTX-343 | |||||||
| LPA1R Antagonist | Peripheral | Wholly-owned | ||||||
| close x | PIPE-307 | |||||||
| M1R Antagonist | RRMS | In Collaboration with Johnson & Johnson | ||||||
| close x | ||||||||
| M1R Antagonist | Depression | In Collaboration with Johnson & Johnson | ||||||
| close x | Calpain | |||||||
| Calpain Inhibitor | Undisclosed | Wholly-owned | ||||||
| close x | ||||||||
| LPA1R Antagonist
IPF | ||||
| Discovery | Preclinical | Phase 1 | Phase 2 | Phase 3 |
|---|---|---|---|---|
Wholly-owned | ||||
| LPA1R Antagonist
Progressive | ||||
| Discovery | Preclinical | Phase 1 | Phase 2 | Phase 3 |
Wholly-owned | ||||
| LPA1R Antagonist
Chronic Pain | ||||
| Discovery | Preclinical | Phase 1 | Phase 2 | Phase 3 |
Wholly-owned | ||||
| LPA1R Antagonist
Peripheral | ||||
| Discovery | Preclinical | Phase 1 | Phase 2 | Phase 3 |
Wholly-owned | ||||
| M1R Antagonist
RRMS | ||||
| Discovery | Preclinical | Phase 1 | Phase 2 | Phase 3 |
In Collaboration with Johnson & Johnson | ||||
| M1R Antagonist
Depression | ||||
| Discovery | Preclinical | Phase 1 | Phase 2 | Phase 3 |
In Collaboration with Johnson & Johnson | ||||
| Calpain Inhibitor
Undisclosed | ||||
| Discovery | Preclinical | Phase 1 | Phase 2 | Phase 3 |
Wholly-owned | ||||
* Partnered
**Wholly-owned
LPA1R Antagonist
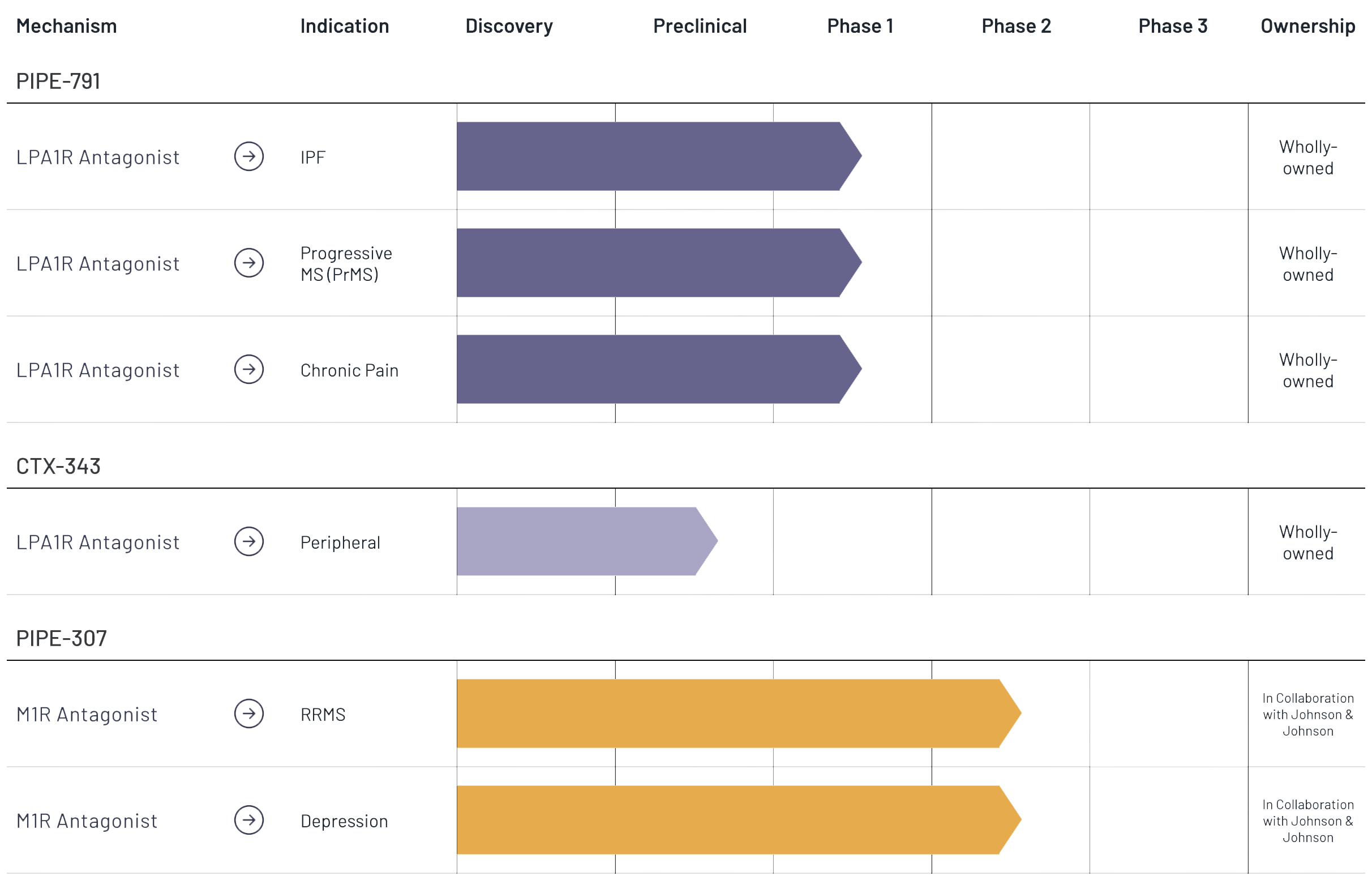
Our wholly-owned lead asset, PIPE-791, is a novel, brain penetrant, small molecule antagonist of the lysophosphatidic acid 1 receptor (LPA1R) in development for the potential treatment of idiopathic pulmonary fibrosis (IPF). We have initiated a Phase 1b open-label trial in IPF to measure lung receptor occupancy by positron emission tomography (PET) imaging, which will inform dose selection for future clinical development in Phase 2a trials.
LPA1R Antagonist
Our wholly-owned lead asset, PIPE-791, is a novel, brain penetrant, small molecule antagonist of the lysophosphatidic acid 1 receptor (LPA1R) in development for the potential treatment of progressive multiple sclerosis (Progressive MS). We have initiated a Phase 1b open-label trial in Progressive MS to measure brain receptor occupancy by positron emission tomography (PET) imaging, which will inform dose selection for future clinical development in Phase 2a trials.
LPA1R Antagonist
Our wholly-owned lead asset, PIPE-791, is a novel, brain penetrant, small molecule antagonist of the lysophosphatidic acid 1 receptor (LPA1R) in development for the potential treatment of chronic pain. We expect to launch an exploratory Phase 1b, randomized, double-blind, placebo-controlled, crossover, multi-center study in the first quarter of 2025.
LPA1R Antagonist
In January 2024, we nominated and began preclinical studies for CTX-343, a peripherally restricted (unable to cross the blood-brain-barrier) LPA1R antagonist. CTX-343 represents the third internally development candidate to be generated from our drug discovery platform. We expect to submit an Investigational New Drug Application (IND) to the U.S. Food and Drug Administration (FDA) for CTX-343 in 2025.
M1R Antagonist
PIPE-307 is being developed pursuant to a global license and development agreement with Janssen Pharmaceutica NV, one of the Janssen Pharmaceutical Companies of Johnson & Johnson.
M1R Antagonist
PIPE-307 (JNJ-5120) is being developed pursuant to a global license and development agreement with Janssen Pharmaceutica NV, one of the Janssen Pharmaceutical Companies of Johnson & Johnson.
Calpain Inhibitor
We have developed a selective calpain inhibitor which was recently moved into preclinical studies. Calpain is a cysteine protease that requires calcium for activation. Inappropriate regulation of the calpain-calpastatin proteolytic system is implicated in a range of significant human pathological processes, including peripheral neuropathies, fibrosis and chronic neutrophilic inflammation. Consequently, blocking calpain with a selective inhibitor could benefit those affected by these disease states.
PIPE-791
PIPE-791 – IPF
Our wholly-owned lead asset, PIPE-791, is a novel, brain penetrant, small molecule antagonist of the lysophosphatidic acid 1 receptor (LPA1R) in development for the potential treatment of idiopathic pulmonary fibrosis (IPF). We have initiated a Phase 1b open-label trial in IPF to measure lung receptor occupancy by positron emission tomography (PET) imaging, which will inform dose selection for future clinical development in Phase 2a trials.
PIPE-791 – Progressive MS (PrMS)
Our wholly-owned lead asset, PIPE-791, is a novel, brain penetrant, small molecule antagonist of the lysophosphatidic acid 1 receptor (LPA1R) in development for the potential treatment of progressive multiple sclerosis (Progressive MS). We have initiated a Phase 1b open-label trial in Progressive MS to measure brain receptor occupancy by positron emission tomography (PET) imaging, which will inform dose selection for future clinical development in Phase 2a trials.
PIPE-791 – Chronic Pain
Our wholly-owned lead asset, PIPE-791, is a novel, brain penetrant, small molecule antagonist of the lysophosphatidic acid 1 receptor (LPA1R) in development for the potential treatment of chronic pain. We expect to launch an exploratory Phase 1b, randomized, double-blind, placebo-controlled, crossover, multi-center study in the first quarter of 2025.
CTX-343
In January 2024, we nominated and began preclinical studies for CTX-343, a peripherally restricted (unable to cross the blood-brain-barrier) LPA1R antagonist. CTX-343 represents the third internally development candidate to be generated from our drug discovery platform. We expect to submit an Investigational New Drug Application (IND) to the U.S. Food and Drug Administration (FDA) for CTX-343 in 2025.
PIPE-307
PIPE-307 – RRMA
PIPE-307 is being developed pursuant to a global license and development agreement with Janssen Pharmaceutica NV, one of the Janssen Pharmaceutical Companies of Johnson & Johnson.
PIPE-307 – Depression
PIPE-307 (JNJ-5120) is being developed pursuant to a global license and development agreement with Janssen Pharmaceutica NV, one of the Janssen Pharmaceutical Companies of Johnson & Johnson.

